Module 6: Vaccination
Learning Objectives
- Differentiate bacteria and viruses.
- Understand the basics of how vaccinations work.
- Understand the two basic types of Immunity.
- Identify the Core and Non-Core Vaccines.
- Understand the use of titers.
What are Bacteria & Viruses?

Bacteria
Bacteria are prokaryotes—single-celled organisms that lack a nucleus. They may be motile or non-motile, which defines their ability to move. Some bacteria use photosynthesis or other synthetic processes to produce food. Most, however, absorb nutrients from the environment; many of which may cause diseases.
Virus
Viruses, on the other hand, are submicroscopic organisms that have protein coats around their genetic codes (DNA or RNA strand). Some viruses are encased in a membrane known as an envelope, which may include spikes on its surface to aid adhesion to a host cell after invasion.
A host cell's metabolism is taken over by the viral strand, which instructs it to multiply the viral material. The virus will then direct the host cell to burst after it has created enough replicates, unleashing the virus in order for it to further replicate in other host cells.
In several circumstances, the host is not harmed by the viral replication cycle. When a high number of host cells in a certain tissue are killed, disease develops, causing bodily functions to be disrupted.
Vaccines
Edward Jenner, later Louis Pasteur, pioneered vaccination, which spread throughout the 19th and 20th centuries. The observation of cross-immunity between two illnesses, cowpox and smallpox, paved way for the formulation of vaccines for different diseases.
To make a vaccine, pathogen isolation, culture, and attenuation or inactivation became a true science. It created the notion of "vaccinology" by combining clinical and biological effectiveness studies with analyses of adverse effects.
Vaccination is the most significant preventative approach for infectious disease prevention. Some of these diseases are more serious than others. As a result, the terms "core" and "non-core" have been used to distinguish between critical vaccinations and those that are not.
Core vaccinations are those that should be given to all dogs. These prevent the animals from acquiring diseases such as canine distemper virus (CDV), canine adenovirus 2 (CAV-2), canine parvovirus type 2 (CPV-2), and rabies — the four main vaccinations for dogs in North America.
Rabies vaccines are also mandated by law in several countries and are necessary for international travel.
Non-core vaccinations depend on a veterinarian's rigorous risk assessment in cooperation with the dog owner. Risks regarding these vaccines vary depending on the dog's lifestyle, vaccination used, geographic region, local environment, and degree of exposure to other dogs.
Unit 1: Two Types of Immunization

Passive Immunization
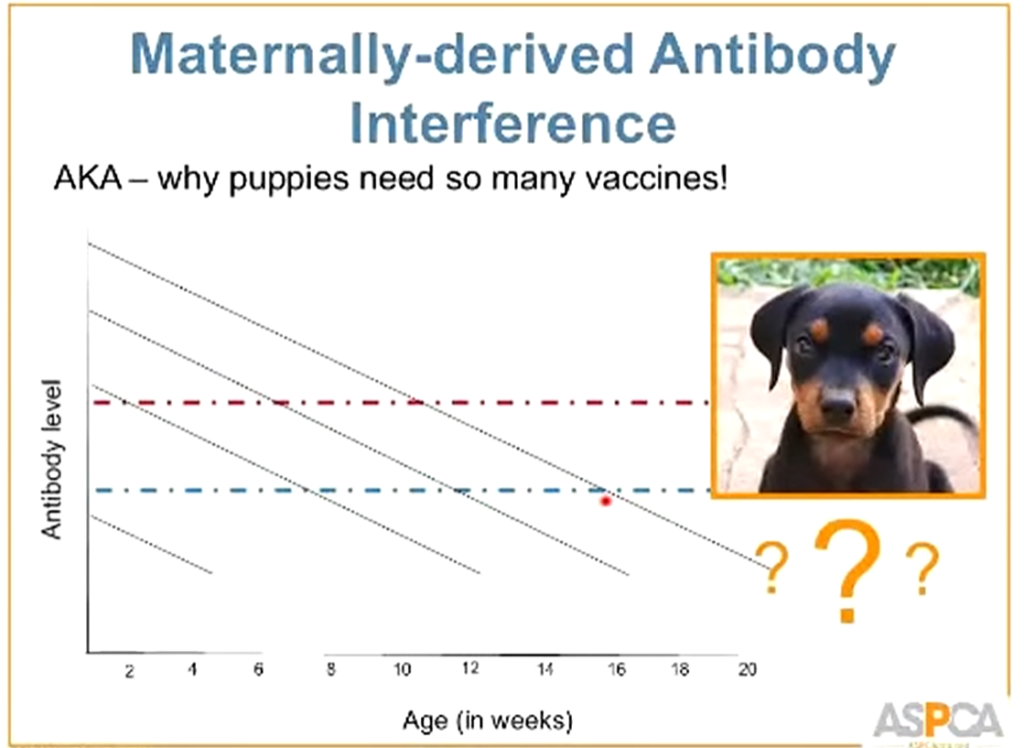
Passive immunity is when a dog is given antibodies to disease from their mother's colostrum or, less frequently, by injection during treatment.
Antibodies obtained from passive immunity will neutralize vaccines before they can serve their purpose.
This is why you need to give puppies frequent vaccinations.
There is a window of time where the maternal antibodies are not enough to protect against a disease, yet still present enough to neutralize a vaccine.
Active Immunization
Compared to passive immunization, dogs will produce their own immunity. Active immunization has a larger number of significant benefits. These include longer periods of immunity and recall and amplification of immune response due to repeated antigen injections or following infection exposure. Therefore, a perfect vaccine for active immunization should provide long-lasting, robust protection. The vaccination should have no unfavorable side effects to produce said strong immunity.
Ideal Vaccine:
- Inexpensive
- Stable
- Adaptable to mass vaccination—should elicit an immunological response distinct from that induced by natural infection, allowing for simultaneous vaccination and eradication.
Unit 2: Core Vaccines
Canine Parvovirus (CPV)

Canine Parvovirus (CPV)—a highly infectious and fatal virus, mostly affects the gastrointestinal system. CPV is transmitted between dogs through direct and indirect contact (fomite). After exposure to infected feces, the virus enters the host through the oronasal pathway. The viral replication then begins in the oropharyngeal lymphoid tissue and subsequently spreads to the mesenteric lymph nodes, thymus, and small intestine. The time between exposure and the onset of clinical illness varies between 4 and 14 days.
Canine parvovirus-2 (CPV) can survive for longer periods of time in the environment, contributing to its high morbidity among vulnerable, exposed dogs. While most dogs will survive infection with appropriate treatment, the resources necessary for isolation and treatment are significant, thus it continues to be one of the most significant infectious diseases.
Pathogenesis
CPV replicates in the epithelium of rapidly dividing cells of small intestinal crypts. In normal dogs, when the epithelium matures, it migrates from intestinal crypts to the villi where nutrient absorption occurs. CPV destroys the germinal epithelium leading to intestinal function impairment.
After infection, viremia occurs one to five days and fecal viral shedding begins around four to five days after. Shedding lasts for 7-10 days but can persist for several weeks. However, when the clinical signs (specifically, diarrhea) resolve, viral shedding starts to lessen.
Clinical Signs
- Acute enteritis
- Vomiting – often severe
- Diarrhea – may or may not be hemorrhagic, often severe
- Depression
- Lethargy
- Severe dehydration
- Abdominal pain
- Fever – for more severe cases
Concurrent diseases like Coronavirus, Canine Distemper virus, intestinal parasites or other pathogens can worsen the clinical signs.
Diagnosis:
- Fecal enzyme-linked immunosorbent assay (ELISA) antigen testing: highly specific, variable sensitivity data
- Fecal polymerase chain reaction (PCR) testing: higher sensitivity than ELISA test; however, ELISA is more practical – making it the first choice for testing
In cases where a pet is highly suspected to be infected with this virus and its ELISA result comes out negative, it is recommended to conduct a re-test. Certain factors that can lead to false negative ELISA results include low fecal viral load, mild clinical symptoms and high fecal antibodies.
Prevention
In order to effectively manage the spread of CPV, it is important to lower both the risk of an individual animal becoming infected and the risk of exposure.
Vaccination
Vaccination against CPV can start as early as four weeks of age to support the puppy from decreasing maternal antibodies. Revaccination should be done every 2 weeks until 20 weeks of age. When discontinued early, the chances of puppies getting infected with the virus increase substantially. For dogs over 20 weeks old, initial vaccination with a booster shot after 2-3 weeks is recommended.
Sanitation
CPV is a non-enveloped virus, thus it can survive in the environment longer. Since the main mode of the viruses’ transmission is feco-oral, thorough cleaning and proper disposal of fecal waste are very important. This process reduces contamination from dogs showing no clinical signs that might be shedding the virus. When cleaning the kennel, degreaser and detergent can be used to remove organic wastes.
Disinfectants:
- Readily available disinfectant
- Accelerated hydrogen peroxide
- 1:32 dilution for 10-minute contact time
- 1:16 dilution for 5-minute contact time
- Ideal choice against CPV in shelters
- Retains some efficacy in the face of organic matter
- Safe and less noxious than bleach
- Potassium peroxymonosulfate
- 1% dilution for 10-minute contact time
- Retains a small degree of efficacy in the face of organic matter
- Works against CPV
- Safe and less noxious than bleach
- Stable for seven days
- Bleach
- 1:32 dilution with 10-minute contact time
- Inexpensive and effective against CPV
- Stable for 24 hours
- No efficacy in the face of organic debris
- Can also be very noxious for staff and animals
Sanitation:
- To avoid contamination, sanitation supplies should be restricted to CPV isolation area
- Thorough cleaning and disinfection of contaminated areas are vital to prevent the spread of the virus
- Common use areas that were exposed with infected animals should be disinfected properly and regularly
- Disinfecting exposed areas multiple times before use is a good practice
- Wash bowls, feeders and other dishes within the isolation area with proper disinfectant
- Dispose contaminated beddings and toys
If CPV exposure happens on grass or dirt, it might be exceedingly difficult to completely disinfect an area. CPV may survive outdoors for up to five months and much longer when shielded from the sun or drying. The best way to deal with contaminated grass and dirt is to avoid exposing extremely susceptible dogs, like puppies, in the future.
Biosecurity
Considering CPV's strong ability to survive outside of the host, it is simple for it to infect both the environment and exposed fomites. A significant factor in spread of CPV is indirect viral transmission through fomites, which can be decreased by effective biosecurity measures.
- Proper hand hygiene, which includes hand-washing and changing of gloves after handling any dog, is important.
- To help lessen the chances of indirect disease transmission, constant and close monitoring for signals of illness or infection is recommended.
- When handling dogs infected with CPV, apt use of personal protective equipment (PPE) is critical to prevent the risk of spreading disease.
- To prevent the spread of CPV from contaminated floors, it is recommended that shoe covers or dedicated isolation footwear be used. Note that the use of foot baths is not effective against the spread of CPV.
- Used PPEs must be removed and handled with utmost care and be disposed of accordingly before leaving an isolation area.
Canine Distemper Virus (CDV)

Canine distemper virus (CDV) is an enveloped virus causing systemic or nervous problems, mainly respiratory, gastrointestinal signs, and immunosuppression. Transmission is through inhalation.
Diagnosis
Diagnosis is made by identifying the virus in a clinical sample using the reverse-transcriptase polymerase chain reaction (rt-PCR) on whole blood, tissue or conjunctiva swab, cerebrospinal fluid (CSF), or urine. The gold standard for diagnosis is virus isolation, which can help in viral infection at low levels.
Prevention
Vaccination is the primary method to control the disease. Vaccines currently available contain Onderstepoort or Lederle strains. Immunity can last for years after recovering from natural infection or after receiving a booster shot.
Vaccination
CDV vaccination can start as early as 6-8 weeks of age; this is best continued with booster shots every 2-4 weeks until the puppy is at least 16 weeks old. A single attenuated live or recombinant vaccine may be enough to elicit protection in naive pups or adults. A triennial booster dose for distemper is advised for older dogs that have received vaccinations.
Canine Adenovirus-2 (CAV-2)

Canine adenovirus-1 (CAV-1) and canine adenovirus-2 (CAV-2) are two adenoviruses that can cause disease in dogs. CAV-1 is the cause of infectious canine hepatitis (ICH), while CAV-2 is one of the numerous pathogens that cause the network of canine infectious respiratory diseases (CIRD).
Due to the close antigenic relationships between the two adenoviruses and the cross-protection present, CAV-2 vaccinations also provide protection against ICH. Although modified live CAV-1 vaccines have been demonstrated to be successful, it has also shown frequent cause of vaccine-associated adverse events (VAAEs), including corneal edema (blue eye) and interstitial nephritis. As a result, modern vaccines include modified live CAV-2 that is less likely to cause VAAEs and offers enough defense against CAV-1 for at least three years.
Necrohemorrhagic hepatitis, corneal edema (blue eye), uveitis, and/or interstitial nephritis are symptoms of ICH, which has a significant mortality.
Rabies

The rabies virus is a single-stranded RNA virus with an envelope. Rabies has the highest mortality rate of all known infectious diseases.
Transmission
Typically, a rabid animal's bite transmits the virus. When virus-filled saliva is deposited into mucous membranes, it may potentially spread. Theoretically, scratches could spread the virus if a dog’s claws are tainted with recently infected saliva; but, this is an extremely rare scenario among people.
In other cases, rabies has been transmitted through corneal and internal organ transplantation, transplacentally, through aerosolization in caves and other enclosed areas, and through ingestion of infected tissues. The rabies virus is not efficiently spread by fomites because it is easily destroyed by UV light, dehydration, and the majority of disinfectants.
Clinical Signs
A physical exam alone seldom identifies rabies. Initial clinical symptoms may resemble those of other illnesses and not appear neurologic. The most common finding would be a clinical course that gradually gets worse over hours or days—with an accumulation of symptoms that eventually point to neurologic causes. The animal will not benefit from therapy, and typically dies within ten days. The most crucial pre-mortem clinical tool is close observation with the patient kept in isolation.
Incubation periods vary greatly. Though majority of dogs and cats show clinical symptoms within six months post-exposure, others become sick in as little as ten days. Three to twelve weeks is the common incubation time.
The prodromal phase, furious (excitatory or encephalitic) phase, dumb (paralytic) phase, and death may be used to categorize the clinical indications of rabies. Clinical signs, however, do not specifically follow an order. Phases might not exist or might overlap. The majority of cases are characterized by a notable furious phase, a brief paralytic phase, followed by respiratory arrest and eventual death.
Prodromal phase clinical symptoms are nonspecific:
- Fever
- Lethargy
- Dehydration
- Anorexia
- Vomiting or diarrhea
- Withdraw and hide, or may appear to become more sociable than usual
- Licking or chewing at the site of the original bite wound
- Piloerection, drooling, and dilated pupils
Clinical indicators of furious (excitatory) phase include the following:
- Restlessness
- Hyperreactivity to sounds, movements, and touch
- Bizarre behavior
- Vocalization
- Aggression
- Grand mal seizures
- Fine muscle tremors, especially in cats
Clinical indicators of dumb (paralytic) phase include the following:
- Persistent lethargy
- Cranial nerve deficits
- Ataxia
- Stiff or hunched gait
- Paresis
Rabid animals may display a mix of excitatory and paralytic symptoms, which include: confusion, anxiety, restlessness, and weakness. Some animals may die suddenly. Majority of rabid animals experience widespread paralysis, respiratory arrest, and death.
Diagnosis
In a clinically unwell, living animal, there is currently no proven test for diagnosis of rabies.
- The direct fluorescent antibody (DFA) test, which is carried out on brain tissue, is the gold standard for rabies diagnosis. When human rabies exposure is a possibility, this test—which is nearly always carried out by a public health laboratory—must be performed.
Head or entire brain must be submitted for DFA test for majority of species. Only personnel who have been trained and given a rabies vaccination should perform decapitation or debraining.
- Some laboratories use immunohistochemistry (IHC) to test fixed brain tissue for the presence of rabies. Negri bodies may be visible through histopathology in rabies-infected neurons, however this is not always the case.
Unit 3: Non-Core Vaccines
Bordetella (Kennel Cough)

Kennel cough syndrome is a condition connected to a number of pathogens – among are:
- Bordetella bronchiseptica
- Canine adenovirus type 2 (CAV2)
- Canine parainfluenza virus (CPIV)
- Canine distemper virus (CDV)
However, Bordetella bronchiseptica is likely to be the primary cause. The clinical manifestation is tracheitis, which is typically self-limiting but can also progress to bronchitis or pneumonia. Because it can affect respiratory system of dogs regardless of age, Bordetella bronchiseptica, CAV2, and CPIV are extremely contagious and frequently present when dogs are housed together. The dog often recovers well after developing nasal and tracheal inflammation that lasts 5–14 days.
Clinical Signs
- Cough
- Sneezing and nasal discharge
- Depressed but still eating
- Retching after coughing
By isolating the infected animal and increasing kennel ventilation and disinfection procedures, transmission from dog to dog can be reduced. Dogs entering boarding facilities are generally recommended to receive a combined vaccine that contains both the CAV2 and CPIV.
Leptospirosis

Leptospirosis is a bacterial zoonotic illness that can affect both people and animals. Serovars are used to categorize leptospiral organisms based on the way that antibodies react to surface proteins. Serovars are further divided into serogroups that share similar antigens. The pathogenic species Leptospira interrogans has various serovars that can cause canine leptospirosis.
Several serovars have been connected to canine leptospirosis including:
- Grippotyphosa (serogroup Grippotyphosa)
- Pomona (serogroup Pomona)
- Bratislava (serogroup Australis)
Clinical Signs
Acute kidney injury (AKI), liver disease, vasculitis, and uveitis are a few of the symptoms of leptospirosis in dogs, which range from acute disease to subclinical infection.
Common symptoms of acute or subacute leptospirosis:
- Lethargy
- Anorexia
- Fever
- muscle pain
- abdominal pain
- signs of shock
- Dehydration
- Vomiting
- Diarrhea
- Bleeding
- Jaundice
- Polyuria
- Oliguria
- Anuria
Diagnosis
Leptospirosis diagnostic tests focus on detecting the organism or finding an antibody response in an affected dog. It is advised to use both kinds of tests to increase the likelihood of correctly diagnosing this zoonotic disease. The clinical signs, clinicopathologic findings, and immunization history of the patient must all be taken into consideration when interpreting the findings of any diagnostic test.
- The most used antibody detection test for the leptospirosis diagnosis is microscopic agglutination test (MAT).
- Enzyme-linked immunosorbent assay (ELISA), which detects antibodies, is a more recent serologic assay for the diagnosis of canine leptospirosis. These tests, which provide qualitative positive or negative antibody results, are said to have advantages over MAT tests such as reduced cost and faster turnaround time.
Canine Corona Virus (CCoV)

Canines frequently contract canine coronavirus (CCoV), which has been linked to high frequency of diarrhea in puppies. After being exposed to with infected feces, dogs get sick. One to four days make up the brief incubation phase. The virus is usually excreted in the feces of infected dogs for three to fourteen days following infection, while there have been reports of viral shedding up to six months after infection. Most infections are self-limiting, and the recommended treatment is supportive care.
Clinical Signs:
- Vomiting
- Diarrhea
- Lethargy
- Inappetence
Unit 4: Vaccination Guideline
Table 3. Canine Vaccination Guideline
| Vaccine | Initial Vaccination (Puppy) | Initial Vaccination (Adult) | Revaccination |
| Core Vaccines | |||
| Canine Parvovirus-2 (CPV-2, MLV) | 6-8 weeks of age then every 2-4 weeks until 16 weeks of age or older | Two doses 2-4 weeks apart are generally recommended by manufacturers, but one dose of MLV is considered protective | Booster at either 6 months or 1 year of age, then not more often than every 3 years |
| Canine Distemper Virus (CDV, MLV) | |||
| Canine Adenovirus-2 (CAV-2, MLV) | |||
| Rabies (killed) | One dose at 12 weeks of ageIf vaccinated earlier than 12 weeks old, the puppy should be revaccinated at 12 weeks of age. In high risk areas, a second dose may be given 2-4 weeks after the first. | Single dose | Booster at 1 year of ageCanine rabies vaccines with either a 1- or 3-year DOI are available. Timing of boosters are determined by this licensed DOI, but in some areas may be dictated by statute. |
| Non-core Vaccines | |||
| Bordetella bronchiseptica (intranasal) | Single-dose as early as 3 weeks old | single-dose | Annually or more often in very high-risk animals |
| Leptospira interrogans (with serogroups canicola and icterohaemorrhagiae, killed) | Initial dose at 8 weeks of age or olderSecond dose is given 2-4 weeks later | Two doses 2-4 weeks apart | Annually |
| Canine Influenza Virus | Two doses 2-4 weeks apart with initial dose at more than 6 weeks of age | Two doses 2-4 weeks apart | Annually |
Read more: https://www.avma.org/sites/default/files/2021-08/State-Rabies-Vaccination-Laws-Chart.pdf
Vaccination Failure
Majority of vaccine failures are caused by improper or insufficient administration of the vaccine or by disregarding the manufacturer's instructions.
Live vaccine may have died as a result of:
- Poor storage
- Use of antibiotics in conjunction with live bacterial vaccines
- Use of chemicals to sterilize the syringe
- Excessive use of alcohol swabs on the skin
Revaccination
The frequency of booster shots depends on how long protection lasts; this in turn is influenced by the antigen content, route of administration, and type of vaccine (killed or live).
Modern, more recent vaccines typically provide long-lasting immunity—mostly requiring re-vaccination every three to four years. Some vaccines may provide lifetime immunity. Additionally, even killed vaccines may provide long-lasting immunity for specific animals.
Most animal vaccines used to be administered annually due to administrative simplicity and the benefit of guaranteeing that an animal receives regular veterinary care. But certain vaccines, like those for canine distemper, produce long-lasting protective immunity, making yearly revaccination inappropriate.
According to studies, most modified live virus (MLV) vaccines cause lifelong sterile immunity in dogs and cats. Contrarily, bacterial immunity lasts considerably shorter and frequently may prevent disease but not infection. Therefore, it is recommended to perform serum antibody assays like rapid test enzyme-linked immunosorbent assay (ELISA) kits, to help determine the recommended intervals between vaccinations.
Persistent antibody titer levels may be used if an animal needs additional protection. These tests can establish whether an animal is a non-responder as well as those that have responded to immunization. It can assess whether an animal with a negative occurrence needs to be revaccinated, decide which vaccines should be administered to an animal with an unknown vaccination history, and help establish whether an immunization after three years is necessary. Thus, if serum antibody assays are feasible, “blind” revaccination should be prevented.
Currently, a dose of vaccine could be cheaper than serological testing. However, testing for antibody status should be preferred over giving a booster vaccination to puppies or adult dogs.
Over-Vaccination of Pets. Why is it happening?
These are two great videos by Dr. Karen Becker and an interview with Dr. John Robb that best summarize what has been going on in the veterinary community for decades.
Keep in mind that these are videos are not meant to argue that vaccinations are bad. The videos are meant to inform the public about the endemic of OVER vaccinating animals for the sake of profit and withholding known information about the side effects of vaccinations. Please do your own research to weigh the pros and cons of different vaccination schedules:
Watch both videos:
This is an intro, done be Dr. Karen Becker, to the source of the dilemma:

This video is a must watch to see what is happening in the trenches to a veterinarian, Dr. John Robb, who is standing up to for his beliefs:

Dr. Ronald Schultz Interviews about Vaccines
Two great interviews done by Dr. Karen Becker to Dr. Ronald Schultz.
Immunology expert Ronald Schultz, PhD, Diplomate ACVIM (American College of Veterinary
Internal Medicine), has spent much of his career studying animal vaccines. Dr. Schultz is
professor and chair of the Department of Pathobiological Sciences at the University of
Wisconsin-Madison School of Veterinary Medicine, and has more than 40 years’ experience in
the field of immunology. His long-time university employment – as opposed to a career in
industry – has provided him with a unique position of neutrality from which to observe the
vaccine industry.

Next interview is in two parts:
Part 1

Part 2



Responses